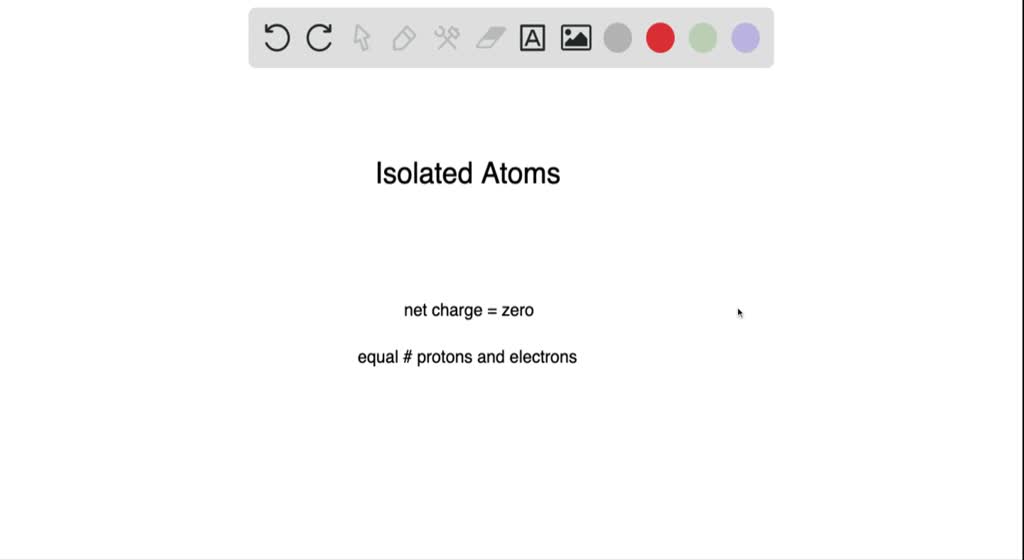
An Isolated Atom Has a Net Charge of
And it should have a neutral charge because theres no other thing around it to balance out net positive or negative charge. An isolated atom is an atom with the same number of electron as protons yeilding a net charge of 0.

Glowing Neon Atom Icon Isolated On Brick Wall Background Symbol Of Science Education Nuclear Physics Scientific Resear Neon Atom Neon Brick Wall Background
Ions are produced when an atom ____ __ ____ _____ Gains or loses electrons.

. Ion A charged entity can be produced called an ion by taking a neutral atom and adding or removing one or more electrons. If the electron derives from a deep non-valence electron level the positive charge is referred to as a core hole. An isolated conductor of arbitrary shape has a net charge.
The total charge added to the system by the creation of the electron and the positron is. When an atom loses one electron the ion formed has a charge of 1 c. Free atoms and the number of electrons assigned to that atom in a Lewis structure.
An isolated conductor of arbitrary shape has a net charge of 10 x 10-6 C. Adam is one that has in that charge of zero because if it stands alone there is no other thing around it to balance out of positive or night charge. For a molecule the net dipole moment is the vector addition of bond moment adn lone pair moment.
Basic Chemistry 7th Edition Edit edition. 1 See answer Add answer 5 pts khatriAbhinav11 is waiting for your help. What is Electric Charge.
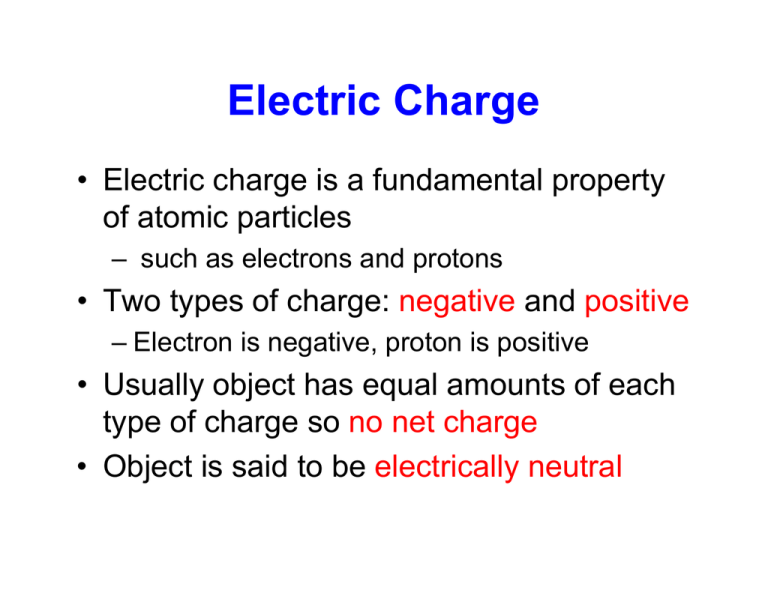
Electric Charge is the property of subatomic particles that causes it to experience a force when placed in an electric and magnetic field. An isolated Adam is expected to have an equal amount of protons and electrons because its expected to have a net charge of zero and isolated atoms will not stands alone. If the atom is an ion or ionizes which is usually the case then it will have a net charge other than zero.
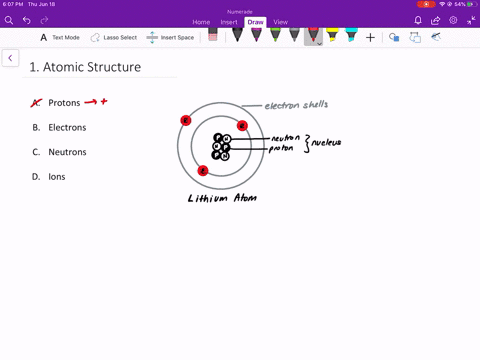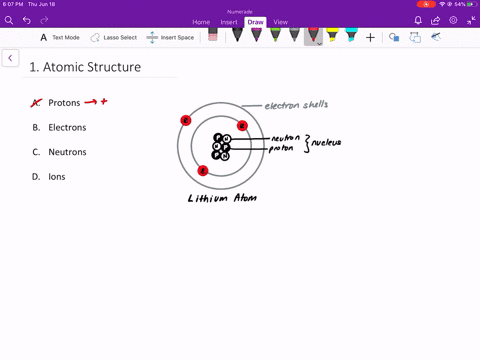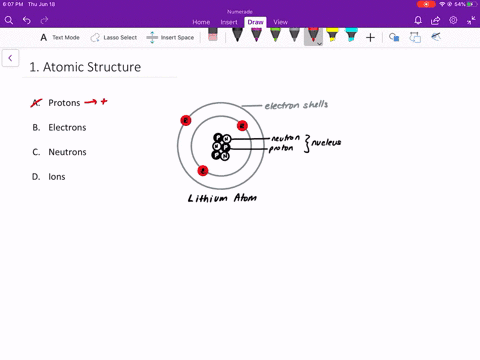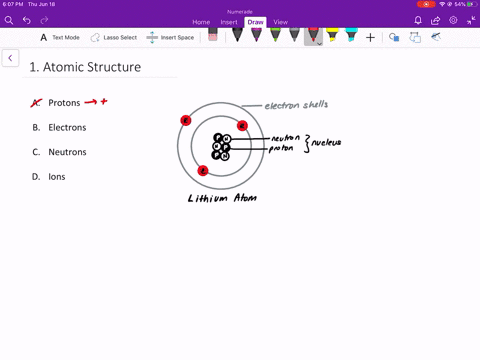
An isolated atom is an atom with the same number of electron as protons yeilding a net charge of 0. An isolated atom has a net charge of zero b. Positive and Negative commonly carried by charge carriers protons and electrons.
This results in an exact balance of positive and negative charges--an atom is a neutral entity--it has a zero net charge. The formal charge is the difference between the number of valence. An isolated conductor has a net charge bartleby An isolated conductor has a net charge of 900 10 6 C and a cavity with a particle of charge q 250 10-6 C.
Atom has a net charge of. The formal charge is the difference between the number of valence electrons in an isolated ie. Get solutions Get solutions done loading.
On the outer surface of the conductor. Apr 21 2015 Law of Conservation of Electric Charge- During any process the net electric charge of an isolated system remains constant is conserved. The point is that the Law Conservation of Charge forces upon us the fact that every time an electron is created from a gamma ray a positron must also be.
A simple ion with a 3 change results when an atom _____ 3 electrons. What is the charge a on the cavity wall and b on the outer surface. Total charge before Total charge after.
There are three types of particles in an atom. Within the systemThe total charge is always the same or the total number of Coulombs will always be the same. Get solutions Get solutions done loading.
The electron has an electric charge of -1 and the positron has an electron charge of 1. When formed an atom loses one electron the ion formed had a change of 1c. This problem has been solved.
A neutral isolated atom has a ground state configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3. Solutions for Chapter 4 Problem 65QP. An isolated atom has a net charge of zero.
Positive ions are called ____. The binding energy of an electron in a particular quantum state of an isolated atom corresponds to the energy to remove the electron an infinite distance from the atom leaving a positively charged ion behind. The question cannot be answered unless the atomic weight is known.
A Number i Units b Number i Units Question thumb_up 100. Electric charges are of two types. There is no universal net charge for atoms.
Physics Secondary School answered an isolated conductor of any shape has a net charge of 10 microcoulomb inside the conductor is a cavity within which is a point charge of 3 microcoulomb what is the charge on the cavity wall. This problem has been solved. An isolated atom has a net charge of ____ Zero.
The properties of an atom in isolation is different from that of individual atoms in relatively close proximity to each other. Looking for the textbook. An atoms net charge is determined by comparing the number of protons and electrons that are in each atom.
Inside the conductor is a cavity within which is a point charge q 30 x 10-6 C. Protons are positive neutron are neutral having no charge and electrons are negative. Protons neutrons and electrons.
In olivine it takes two divalent cations to balance the 4 charge of an isolated tetrahedron. If there is a positive net charge it means that the atom has more. In a neutral atom where the net electric charge is 0 there are an equal number of protons and electrons.
Electricity and Magnetism. So its expected to have a net charge of zero so that means that has an equal number of protons and electrons. Solutions for Chapter 4 Problem 65QP.
Study Guide for ZumdahlDeCostes Introductory Chemistry 7th 7th Edition Edit edition. Protons are positively charged. Assuming you are referring to the charge of an atom the net electric charge is the total charge an atom has.
An ion that has two more electrons outside the nucleus than there are protons in the nucleus will have a ___ charge-2. Because each silicon ion is 4 and each oxygen ion is 2 the three oxygens 6 and the one silicon 4 give a net charge of 2 for the single chain of silica tetrahedra. In pyroxene the one divalent cation 2 per tetrahedron balances that 2 charge.
An isolated atom has a net charge of ________. This is because medium and. So in order for that to happen it has to have the same amount of protons and.

Pi Bonds Pi Bond Bond Molecules

How Many Parties Do You Have On Your Calendar For This Holiday Season It Can Get A Little Craz Red Nosed Reindeer Christmas Reindeer Merry Christmas Animation
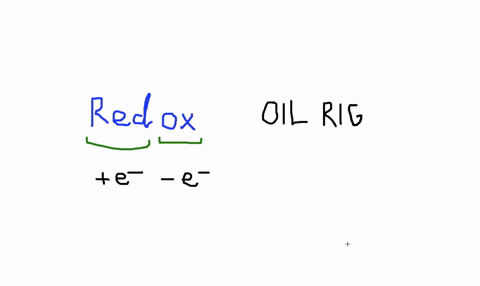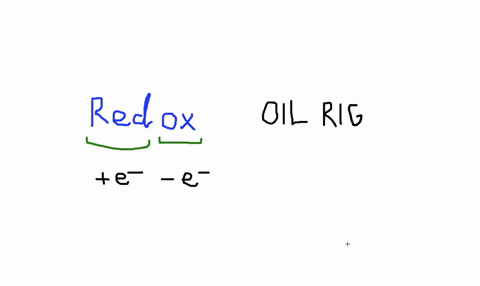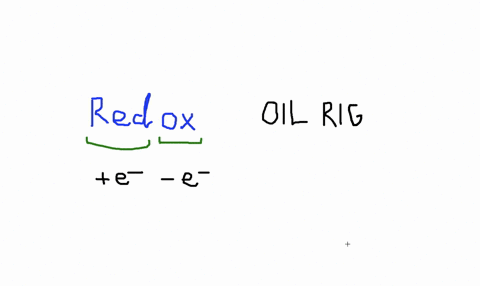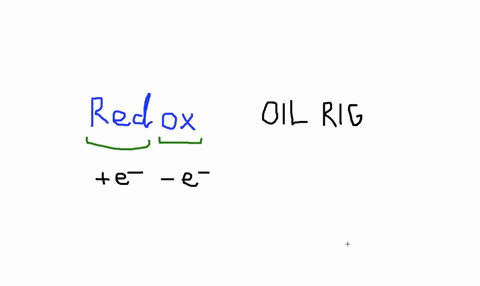
Solved An Isolated Atom Has A Net Charge Of

The Formal Charge Is The Difference Between The Number Of Valence Electrons In An Isolated I E Free Atoms And The Number Of Electrons Assigned To That Atom In A Lewis Structure For
Solved An Isolated Atom Has A Net Charge Of

Solved An Isolated Atom Has A Net Charge Of

Physics React Science Bold And Thin Black Line Icon Set React Icons Line Icons Science Icons Png And Vector With Transparent Background For Free Download Line Icon Science Icons Icon Set
Solved An Isolated Atom Has A Net Charge Of

Deprotonation Of Alpha C Mcat Alpha Electrons

Why Does No3 Have A Charge Of 1 Shouldn T The Charges Add Up To 9

Black Realistic Geometric Rose Flower Triangle Temporary Tattoos Sticker Moon Tattoo Body Art Plum Blossom Fake Tatoos Triangle Tattoos Deer Tattoo Stag Tattoo

What Is Ai Types Of Artificial Intelligence Seo Website Artificial Intelligence Website Development Company

Abstract Red Atom Done In 3d Isolated Ad Spon Red Abstract Isolated Atom Credit Repair Remove All Repair
Solved An Isolated Atom Has A Net Charge Of
Solved An Isolated Atom Has A Net Charge Of

Potassium Atom Showing Electrons In Their Shells Gcse Chemistry Electrons Chemistry


Comments
Post a Comment